
Recently, Professor Dong Xiaochen’s research team has made important progress in photoimmunotherapy. The achievement was published in the internationally renowned journal Advanced Materials with Jiangsu Normal University as the first corresponding institution, entitled Mitochondrial-Targeted Type I Photodynamic Therapy for Agonist Independent cGAS-STING Activation. Xu Yin, a postgraduate student from the School of Chemistry and Materials Science of Jiangsu Normal University, is the first author of the paper. Professor Dong Xiaochen, Associate Professor Guo Yuxin and Professor Qu Lulu are the co-corresponding authors. The paper was funded by the National Natural Science Foundation of China.
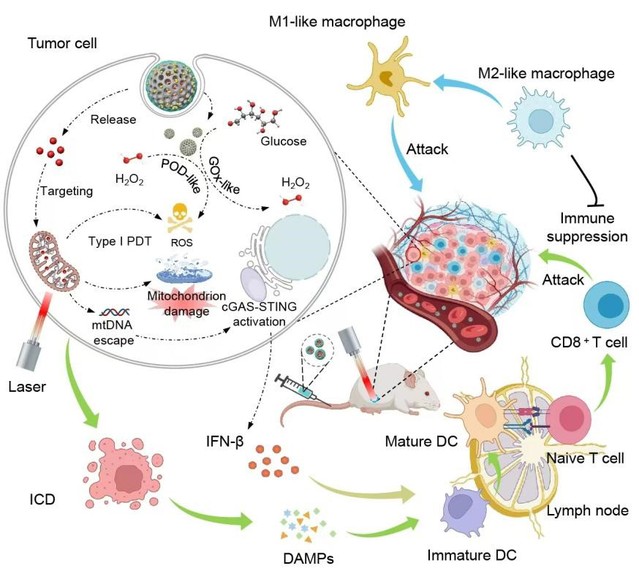
CGAS-STING agonists generally lead to hyperimmunity and systemic toxicity, hindering their immunotherapeutic outcomes. Herein, a mitochondrion-targeted nanoagonist (termed HABH) containing boron dipyrromethene (BODIPY)-derived type I photosensitizer (BDP) and Au nanoparticle-engineered hollow mesoporous silica (HMSN/AuNPs) has been fabricated for light-controlled mitochondrial stress-inducing and agonist-independent cGAS-STING pathway activation. The HABH nanoagonist can actively target tumor tissues and release the mitochondrion-targeted BDP. Under light illumination, BDP achieves type I photodynamic therapy (PDT) in mitochondria, generating massive hydroxyl radicals (•OH) and inducing mitochondrial stress in an oxygen-independent manner, promoting the release of mitochondrial DNA (mtDNA). Simultaneously, the HMSN/AuNPs act as dual nanozymes to derive cascade reactions for •OH production, elevating the intracellular oxidative state, and together with the BDP-induced mitochondrial stress, finally evoking the cGAS-STING pathway and facilitating the release of type I interferon. In the orthotopic breast tumor models, the HABH nanoagonist achieved intratumoral and systemic immunoactivation for eradicating primary tumors and preventing metastasis tumors. Therefore, the constructed mitochondrion-targeted nanoagonist enabled light-controlled and agonist-independent cGAS-STING activation, providing a paradigm for photoimmunotherapy.
Link to the paper: https://doi.org/10.1002/adma.202418894
