Recently, “Formation of ammonia-helium compounds at high pressure” by group of Professor Li Yinwei from School of Physics and Electronic Engineering, Jiangsu Normal University was published in Nature Communications, which is the second time of Professor Li and his group publishing research in NC since 2018 and another breakthrough after their research published in Physical Review Letters in 2020.

High-pressure, a variable quantity independent from temperature and chemical composition in Physics, would produce new physical phenomenon, which especially has an advantage at enriching and developing Condensed Matter Physics. An important source of new materials is structures under high pressure.
“Helium is the most chemically inert element due to its stable closed-shell electronic configuration. Recent studies have indicated that high pressure can induce He to form compounds such as HeN4, Na2He, FeHe, MgOHe, H2OHe, and FeO2He.”
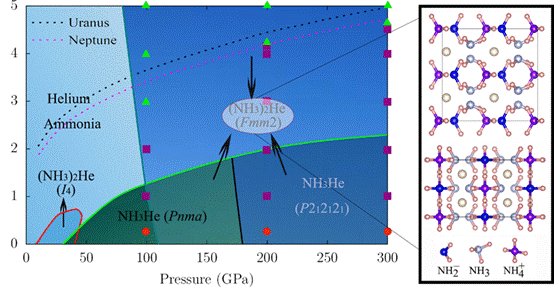
“Uranus and Neptune are generally assumed to have helium only in their gaseous atmospheres. Here, we report the possibility of helium being fixed in the upper mantles of these planets in the form of NH3–He compounds. Structure predictions reveal two energetically stable NH3–He compounds with stoichiometries (NH3)2He and NH3He at high pressures.”
“At low temperatures, (NH3)2He is ionic with NH3 molecules partially dissociating into (NH2)− and (NH4)+ ions. Simulations show that (NH3)2He transforms into intermediate phase at 100 GPa and 1000 K with H atoms slightly vibrate around N atoms, and then to a superionic phase at ~2000 K with H and He exhibiting liquid behavior within the fixed N sublattice. Finally, (NH3)2He becomes a fluid phase at temperatures of 3000 K. The stability of (NH3)2He at high pressure and temperature could contribute to update models of the interiors of Uranus and Neptune.”
